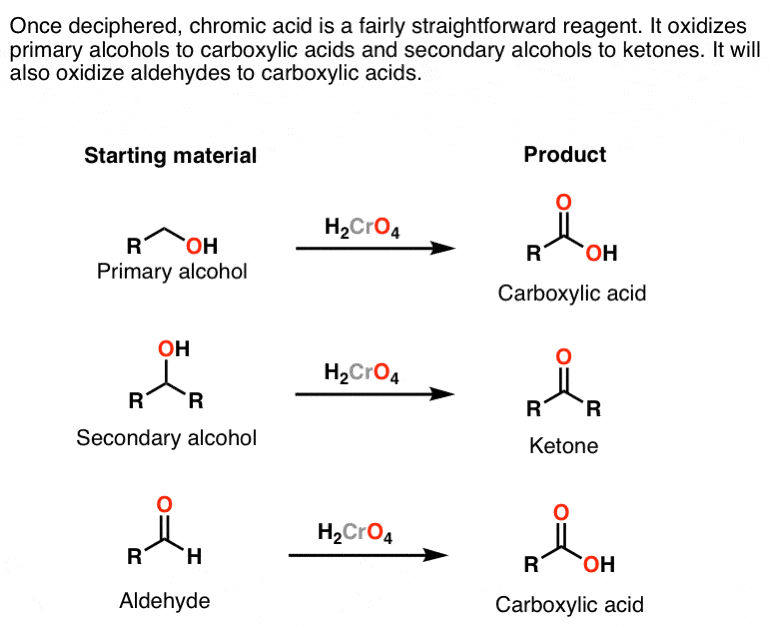
Alcohol Chromic Acid Test
Acetyl Chloride Test. Pg324 The answer is B.
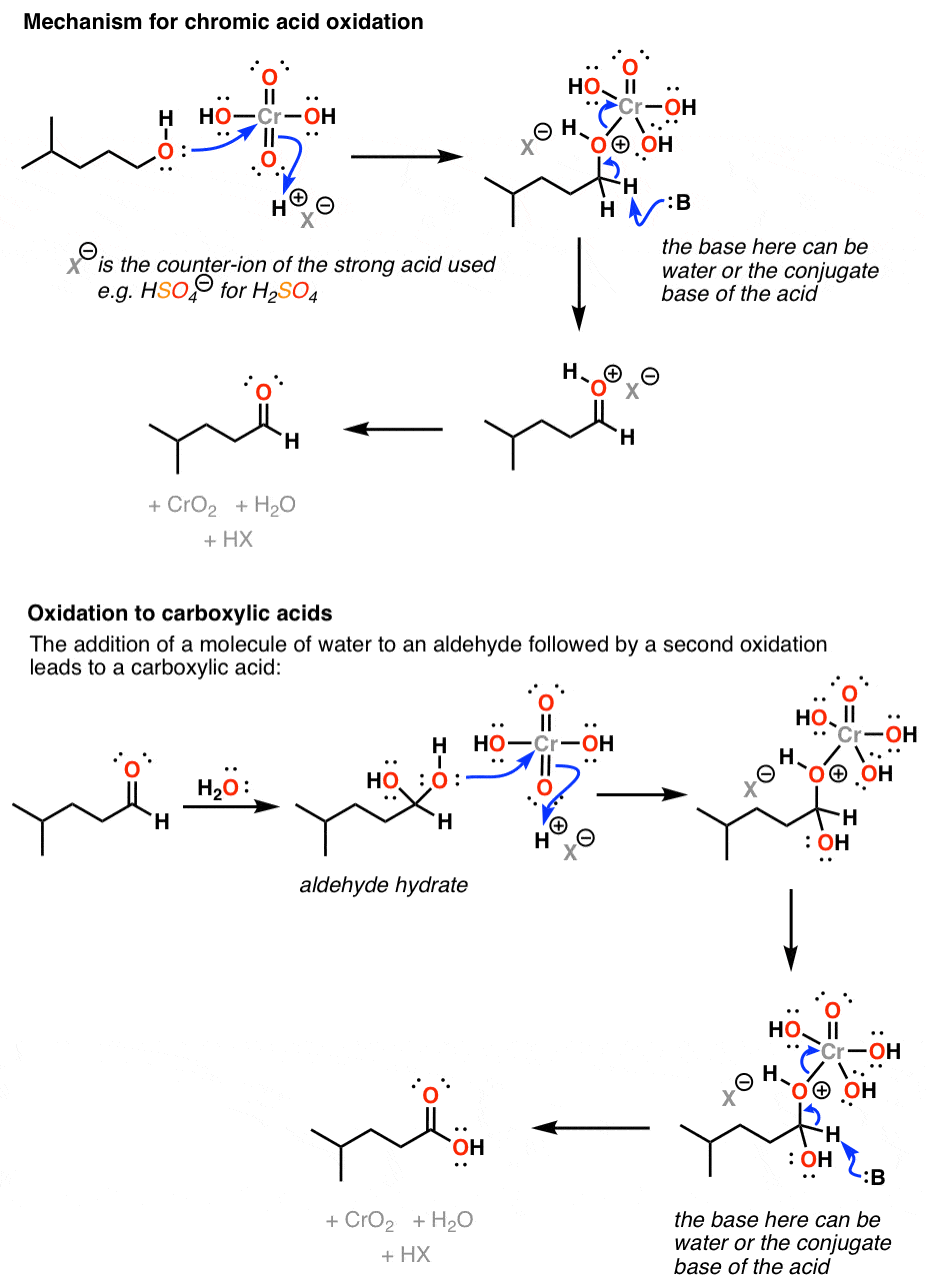
Reagent Friday Chromic Acid H2cro4 Master Organic Chemistry
To each test tube add 10 drops of reagent grade acetone and 2 drops of chromic acid.
. Three drops of the compound to be tested are mixed with 5 drops of acetone and 5 drops of chromic acid solution an orange solution. Tertiary alcohols do not react. Because of the oxidation is signaled by a color change from orange to a blue-green chromic acid is used as a.
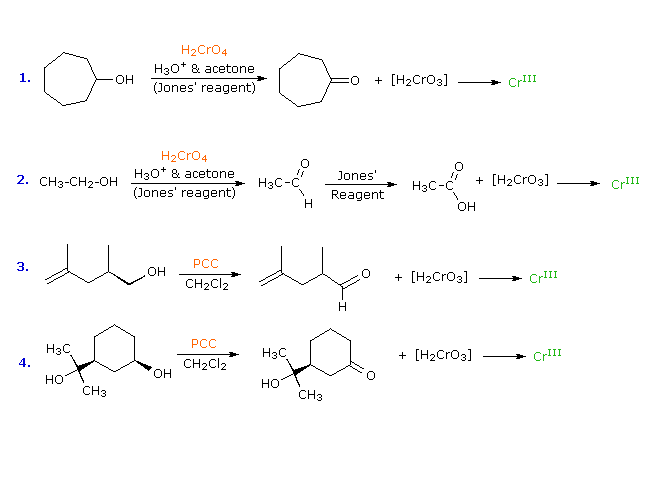
The hydrogen chloride gas reacts with ammonium hydroxide to give white fumes of ammonium chloride. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a carboxylic acid and it will oxidize a secondary alcohol to a ketone. The boiling point was tested to be between 803 to 811.
Identify that alcohol that test positive for Esterification Chromic Acid test Iodoform Test. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Chromic Acid Oxidation This test distinguishes primary and secondary alcohols from tertiary.
Butyl alcohol H 2 CrO 4 3. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. How to perform the test.
Chromic Acid Test - Oxidation of Alcohols Alcohol Color after 2 min Condensed Structural Formula Classification Condensed Structural Formula of Product Ethanol Green 2-Propanol Green Orange 2-Methyl-2- propanol Cyclohexanol Amberbrown Phenol Brown Unknown Amberbrown NA NA NA IronIII chloride Test Alcohol FeCl3 Test Color Ethanol. 1-butanol Primary Alcohol 2-butanol Secondary Alcohol t-butanol Tertiary Alcohol 2. Butyl alcohol H 2 CrO 4 2.
The OH-bearing carbon must have a hydrogen atom attached. Chromic acid test is a qualitative test that can be used to confirm whether a given unknown compound is an aldehyde or not. The qualitative tests for alcohols are i Chromic Acid Test or Jones Oxidation ii Lucas Test using rmZnC.
Alcohols react with acid halides to form esters with the liberation of hydrogen chloride gas. Anhydrous Zinc Chloride in concentrated hydrochloric Acid Lucas Reagent Acetone Chromic Acid reagent Water h20 Sodium hydroxide NaOH Lugols Iodine Methods The first reaction which I performed was the Lucas. M9 Check-In Activity Name of Test Test Solution Reagents Observations CHROMIC ACID n - butyl alcohol H 2 CrO 4 1.
The color changes from orange to green tert. A positive test results in the formation of a blue-green solution from the brown-red color of chromic acid. Materials and Methods Materials 1.
When a primary or secondary alcohol is added to the chromic acid reagent the orange color changes to green or blue. Tertiary alcohol groups are unaffected. The chromic acid test for primary and secondary alcohols exploits the resistance of tertiary alcohols to oxidation.
Possible options are ethyl alcohol n-propyl alcohol isopropyl alcohol sec-butyl alcohol tert-butyl alcohol tert-amyl alcohol. This video provides a demonstration in showing alcohols and aldehydes oxidizes under this test to eventually a carboxylic acid under this test. Remember the loss of the brown-red and the.
Secondary alcohols are oxidized to ketones while the Cr 6 ion in the chromic acid is reduced to Cr 3. The color changes from orange to green sec. When a nonoxidizable substance such as a tertiary alcohol a ketone or an alkane is added to the reagent no.
Place into separate clean dry test tubes 100 x 13 mm labeled as before 5 drops of sample to be tested. Note the color of each solution. Place the test tubes in a 60C water bath for 5 min.
Even though aldehydes give a positive response to this test ketones do not.

Reagent Friday Chromic Acid H2cro4 Master Organic Chemistry

Chromic Acid Test For Alcohols Youtube

Comments
Post a Comment